Current and Future Resources
TRaC requires three technology platforms to help translate clinical ‘omics data from solid tumors into precision drugs that target the DSB response.
The Molecular Analytics Platform is the data integration hub for the entire TRaC program and the base for clinical bioinformatics, molecular dynamics simulations, structural modeling, and machine learning. This infrastructure will allow the team to identify mutational signatures in repair proteins, store and mine structural data, and generate dynamic protein structures to meet the drug discovery needs of the program.

The Target Discovery Platform consisting of three resource labs for precision mapping of altered DNA repair mechanisms in patient-derived cells will help to identify specific components of the damage response that could sustain therapeutic targeting. The Charbonneau Microscopy Facility uses imaging technologies to catalog repair pathway usage through sophisticated DNA repair assays.
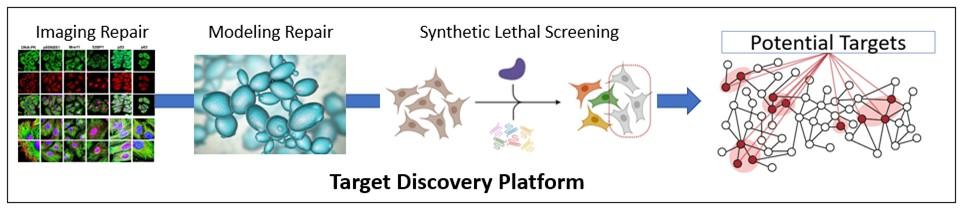
The Yeast Genetics Facility enhances our ability to model repair behavior in the yeast model organism, an essential living test-tube for human repair processes. The TFL Flow Cytometry Core uses synthetic lethality screening technology to identify a molecular Achilles’ heel for major mutational signatures: repair proteins that could cause cell death when “deactivated” with a drug in a specific mutational background. These components, along with innovative gene editing methods, will allow the team to model repair processes a range of cell types, determine how repair mechanisms respond to mutations, and identify repair proteins that drive cell death in clinically relevant genetic backgrounds. The platform will be a major national resource for studying the fundamental processes governing DNA repair.
Finally, to support drug discovery, the TRaC program will establish Canada’s first Integrative Structure Biology Platform, comprised of three major facilities:

Drug design requires the high-resolution structures of repair processes, but no single technology can model protein structure and function at this scale. This platform will determine the structures of whole repair systems using data produced from cryo- electron microscopies, mass spectrometry and artificial intelligence. A combination of methods that overlap in the scales of organization can transform structural biology from a laborious undertaking into a streamlined activity that accurately models the structures of cellular complexes, with no size limit. Structures emerging from this platform will support the computational screening of massive collections of drug-like compounds, to generate “hits” that validate the drug targets and seed the development of new experimental therapeutics with our partners.

